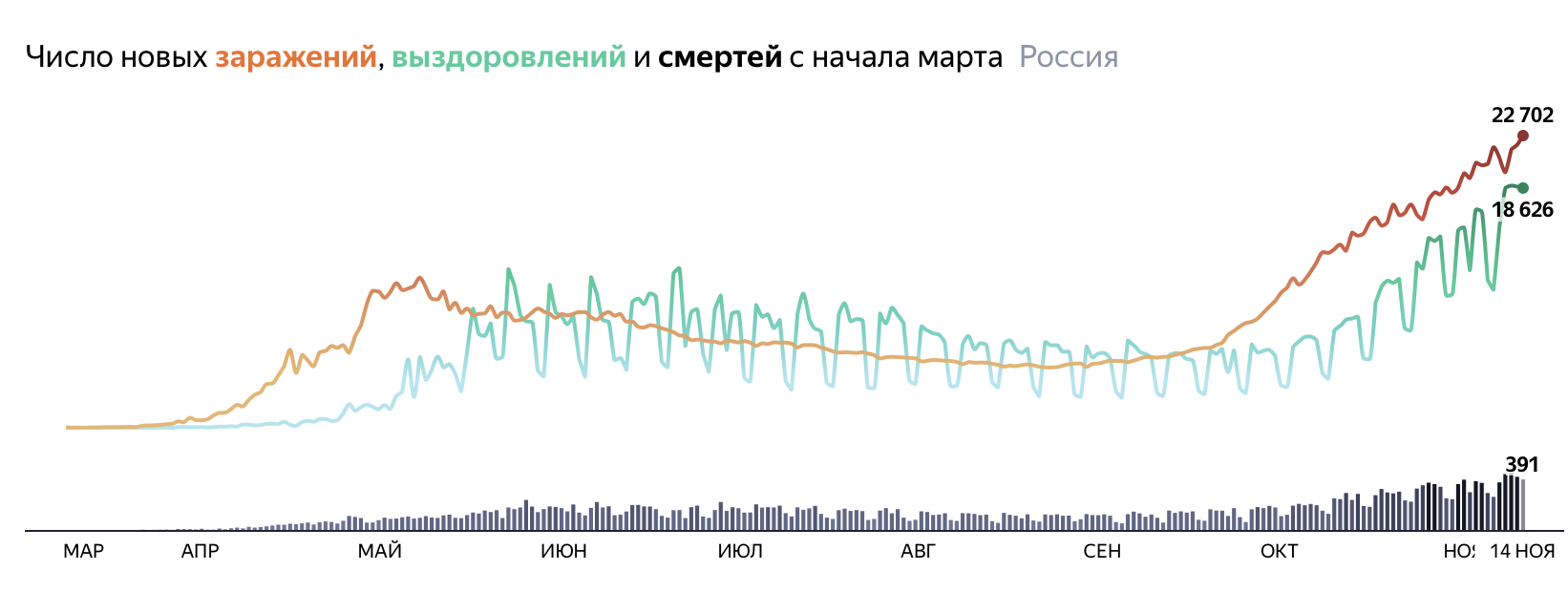
Sensitive tests mean that many who test positive for covid barely carry any virus, making it hard to grasp true reach of disease
Содержание:
- Questions and answers
- Is there an over-the-counter COVID-19 test I can take at home?
- Is there a prescription COVID-19 test I can take at home?
- How long does it take for coronavirus test results to come back?
- Who should get tested for coronavirus?
- When can I be around other people after I tested positive for COVID-19 but had no symptoms?
- Heroes we deserve
- Interpreting antibody tests
- The knowledge of false positives/negatives are directly applicable
- Diagnostic Tests with Alternative Options
- How to get tested
- Call up Guinness World Records
- Why the coronavirus actually kills about 10% of those who become symptomatic
- What about COVID-19?
- A step-by-step example
- Summary
- References
- How false positives occur
- Recommendations
- Types of tests
- Increasing Access to Testing
Questions and answers
Is there an over-the-counter COVID-19 test I can take at home?
Yes. The U.S. Food and Drug Administration (FDA) issued an Emergency Use Authorization (EUA) for an over-the-counter fully at-home diagnostic test for COVID-19. The test is authorized for individuals two years of age or older, including those not showing symptoms.
The home test is a rapid, lateral flow antigen test, a type of test that runs a liquid sample along a surface with reactive molecules. The FDA allows it to be sold in places like drug stores, where a patient can buy it, swab their nose, run the test and find out their results in as little as 20 minutes.
Individuals with positive results should:
- Self-isolate
- Seek additional care from their health care provider
Individuals who test negative and experience COVID-like symptoms should follow up with their health care provider. It is possible to get a negative test result and still be infected with coronavirus.
For more information, see the FDA news release.
Is there a prescription COVID-19 test I can take at home?
Yes. The U.S. Food and Drug Administration (FDA) issued an Emergency Use Authorization (EUA) for an at-home COVID-19 diagnostic self-test. The authorization is for home use with self-collected nasal swab samples in individuals aged 14 and older. This test is currently authorized for prescription use only.
The test works by swirling the self-collected sample swab in a vial that is then placed in the test unit. In 30 minutes or less, the results can be read directly from the test unit’s light-up display.
Individuals with positive results should:
- Self-isolate
- Seek additional care from their health care provider
Individuals who test negative and experience COVID-like symptoms should follow up with their health care provider. It is possible to get a negative test result and still be infected with coronavirus.
For more information, see the FDA news release.
Turnaround time for coronavirus test results is usually less than two days. Approximately two-thirds are returned within a day and more than 85% are available within two days.
This turnaround time includes shipping time. So for labs that process home testing kits, turnaround time may depend on when an individual mails back their kit.
If you haven’t received your test results and it’s been several days, contact your healthcare provider, testing service, or local health department.Read more at California’s COVID-19 Testing Task Force.
You should get tested if you:
- Had with anyone who has tested positive for COVID-19
- Have COVID-19 symptoms
- Get a call from a contact tracer
- Are at high risk
If you think you may have been exposed, call your doctor.
When can I be around other people after I tested positive for COVID-19 but had no symptoms?
If you continue to have no symptoms, you can be with others after 10 days have passed since your test.The CDC has detailed recommendations for people who test positive but have no symptoms.
You should self-isolate from others in your household who have not tested positive. Sleep and stay in a separate room from them, and use a separate bathroom, if possible. Multiple infected people in the same household can use the same room for isolation.
Members of your household should get tested right away. They should quarantine for at least 14 days. Symptoms can develop even after testing negative within 14 days after exposure. Multiple people in the same household should not quarantine in the same room, since some may be infected.
Heroes we deserve
Interpreting antibody tests
Interpretation of test results depends not only on the accuracy of the test itself but also the pre-test probability of infection. This will vary widely depending on the indication for testing: when screening asymptomatic individuals the pre-test probability will be relatively low, for those with suggestive symptoms it is likely to be much higher. We illustrate this with two (fictitious) clinical cases.
Case 1
Anthony is 53, has type 2 diabetes, and a raised body mass index. He works as a security guard in a shopping centre in Norwich. His wife is worried about his risk of exposure to covid-19 at work, and phones the GP surgery requesting an antibody test. He has not had any suggestive symptoms and has no known exposure.
Anthony’s pre-test probability can be estimated based on the population SARS-CoV-2 antibody seroprevalence in his area; in the East of England this is estimated to be around 10%. As he has had no symptoms or known exposure his probability of asymptomatic seroconversion is likely to be lower; for illustrative purposes we estimate his pre-test probability at 5%.
We do not have any data on the accuracy of antibody assays in asymptomatic people on which to base our estimates. We will start by using the average sensitivity of 91.4% and average specificity of 98.7% from the Cochrane review and consider what would change if, as is likely, the test had a lower sensitivity. illustrates the outcomes of testing based on 1000 people like Anthony, with a pre-test probability of 5%. We would expect that 942 people would test negative, of whom four (0.4%) would actually have had covid-19 (false negatives). Considering that the test may well have a lower sensitivity, particularly if the peak incidence and therefore likely time of infection is >35 days ago, this would proportionally increase the false negative rate. If the test made five times as many false negatives (sensitivity of 57%) then this would rise to 20 false negatives (2.1%)—still relatively low numbers owing to the low prevalence. A negative test result would therefore mean Anthony is unlikely to have had covid-19 infection. However, of the 58 people who would test positive, 12 people (21%) would be falsely positive. This is important because a false positive could potentially influence Anthony’s behaviour and adherence to infection control measures. This could be particularly risky as Anthony has an occupational risk of exposure and comorbidities, placing him at higher risk of complications from covid-19. The GP should therefore explain that the test result cannot be used to indicate immunity, and that regardless of the results of testing, Anthony should follow recommended precautions to avoid exposure to SARS-CoV-2. The test result in this case is therefore unlikely to change any advice given to the patient, and has the potential to cause harm through false reassurance.
Fig 1
Infographic showing outcomes of SARS-CoV-2 antibody testing based on 1000 people with a pre-test probability of 5%
The knowledge of false positives/negatives are directly applicable
How do you declare a person COVID-19 positive? After you get a positive result from the test.
But, as we discussed, every test result is uncertain to some extent. So, we cannot actually say with 100% certainty that a person is COVID-19 positive, we can only say with high enough probability. Now, if we cast the testing process in terms of probability, here are a few quantities we can write,
P(COVID-19 positive| test = positive): This denotes the probability that the person is really COVID-19 positive given that the test result is positive. It is called a conditional probability expression. We want to calculate this. Now, if you look at the Bayes’ rule formula above, you will recognize it to be equivalent to the posterior expression P(A|B).
P(test = positive|COVID-19 positive): This is the likelihood P(B|A) in the Bayes’ rule. This is nothing but sensitivity i.e. how many true positives (test results) are there among all the positive cases (in reality).
P(COVID-19 positive): This is the probability of a random person having been infected by the COVID-19 virus. In the domain of medical testing, this is called the ‘prevalence rate’. This term appears in the numerator of the Bayes’ rule ( P(A) in the Bayes’ rule) as the Prior. This is the piece of the information that is not test-specific but needs domain knowledge or broader statistical measure. For COVID-19, experts may say, after pouring over a lot of data from all over the world that the general prevalence rate is 0.1% i.e. 1 out of 1000 people may be infected with the virus. Of course, this number can change based on the country, health system, active social distancing measure, etc. In particular, the antibody-test or the so-called Serological tests can give a good measure of this rate.
P(test=positive): This is the denominator in the Bayes’ rule equation i.e. P(B). This can be calculated as,
P(test=positive) = P(test=positive|COVID-19 positive)*P(COVID-19 positive)+P(test=positive|COVID-19 negative)*P(COVID-19 negative)
Clearly, this calculation takes into account the fact that we can get a positive test result both for a truly infected person or a FALSE POSITIVE for a non-infected person. The term P(test=positive|COVID-19 negative) is simply the FALSE POSITIVE rate calculated from the confusion matrix. The term P(test=positive|COVID-19 positive) is the sensitivity as appearing in the numerator (discussed above).
Therefore, we can see that all the characteristics of a medical test can be readily utilized in a Bayesian calculation.
But there is more to the Bayesian statistics than this!
Diagnostic Tests with Alternative Options
How to get tested
California has partnered with Verily and OptumServe to provide free, confidential testing statewide. Tests are available for everyone, including underserved communities and individuals who are at high risk.
Testing with Verily
Verily provides drive-through testing. To find a Verily testing site near you and make an appointment:
You will need a Google account.
Drive-through testing in Northern California
- Alturas (Modoc County)
- American Canyon (Napa County)
- Antioch (Contra Costa County)
- Atascadero (San Luis Obispo County)
- Bear Valley (Alpine County)
- Bishop (Inyo County)
- Calistoga (Napa County)
- Calpine (Sierra County)
- Ceres (Stanislaus County)
- Chowchilla (Madera County)
- Clearlake (Lake County)
- Coleville (Mono County)
- Corcoran (King County)
- Corona (King County)
- Crescent City (Del Norte County)
- Daly City (San Mateo County)
- Dinuba (Tulare County)
- Downieville (Sierra County)
- Earlimart (Tulare County)
- East Palo Alto (San Mateo County)
- Elk Grove (Sacramento County)
- Exeter (Tulare County)
- Farmersville (Tulare County)
- French Camp (San Joaquin County)
- Fresno (Fresno County)
- Gridley (Butte County)
- Half Moon Bay (San Mateo County)
- Hanford (King County)
- Hayfork (Trinity County)
- Jackson (Amador County)
- Kerman (Fresno County)
- Klamath (Del Norte County)
- Lakeport (Lake County)
- Lee Vining (Mono County)
- Lemoore (King County)
- Lewiston (Trinity County)
- Livingston (Merced County)
- Lone Pine (Inyo County)
- Los Baños (Merced County)
- Loyalton (Sierra County)
- Magalia (Butte County)
- Mammoth Lakes (Mono County)
- Markleeville (Alpine County)
- Merced (Merced County)
- Middletown (Lake County)
- Napa (Napa County)
- Placerville (El Dorado County)
- Pescadero (San Mateo County)
- Porterville (Tulare County)
- Redding (Shasta County)
- Redwood City (San Mateo County)
- Reedley (Fresno County)
- Rio Linda (Sacramento County)
- Sacramento (Sacramento County)
- Salida (Stanislaus County)
- San Bruno (San Mateo County)
- San Jose (Santa Clara County)
- San Mateo (San Mateo County)
- San Rafael (Marin County)
- Santa Rosa (Sonoma County)
- Selma (Fresno County)
- St. Helena (Napa County)
- Stockton (San Joaquin County)
- Susanville (Lassen County)
- Tracy (San Joaquin County)
- Tulare (Tulare County)
- Turlock (Stanislaus County)
- Upper Lake (Lake County)
- Vallejo (Solano County)
- Visalia (Tulare County)
- Wawona (Mariposa County)
- Weaverville (Trinity County)
- Woodlake (Tulare County)
- Woodland (Yolo County)
- Yosemite Valley (Mariposa County)
Drive-through testing in Southern California
- Alpine (San Diego County)
- Anaheim (Orange County)
- Apple Valley (San Bernardino County)
- Bakersfield (Kern County)
- Banning (Riverside County)
- Barstow (San Bernardino County)
- Beaumont (Riverside County)
- Bell (Los Angeles County)
- Blythe (Riverside County)
- Brawley (Imperial County)
- Calexico (Imperial County)
- Coachella (Riverside County)
- Compton (Los Angeles County)
- Costa Mesa (Orange County)
- Delano (Kern County)
- Desert Hot Springs (Riverside County)
- El Centro (Imperial County)
- Fallbrook (San Diego)
- Fontana (San Bernardino County)
- Fullerton (Orange County)
- Gardena (Los Angeles County)
- Hemet (Riverside County)
- Hesperia (San Bernardino County)
- Indio (Riverside County)
- La Habra (Orange County)
- Lake Elsinore (Riverside County)
- Lamont (Kern County)
- Lancaster (Los Angeles County)
- Lemon Grove (San Diego County)
- Los Angeles (Los Angeles County)
- McFarland (Kern County)
- Oceanside (San Diego)
- Ontario (San Bernardino County)
- Palmdale (Los Angeles County)
- Paramount (Los Angeles County)
- Pasadena (Los Angeles County)
- Perris (Riverside County)
- Phelan (San Bernardino County)
- Pico Rivera (Los Angeles County)
- Pomona (Los Angeles County)
- Port Hueneme (Ventura County)
- Riverside (Riverside County)
- Rosamond (Kern County)
- San Jacinto (Riverside County)
- San Juan Capistrano (Orange County)
- Santa Maria (Santa Barbara County)
- Santa Paula (Ventura County)
- Seal Beach (Orange County)
- Shafter (Kern County)
- Valley Center (San Diego County)
- Victorville (San Bernardino County)
- Wasco (Kern County)
Testing with OptumServe
Tests are by appointment only. Find a location near you and make an appointment at:
If you do not have internet access, call 1-888-634-1123.
OptumServe community testing sites serve all individuals who qualify for a test. This includes uninsured, underinsured, undocumented and homeless individuals. You do not need a driver’s license to get this test.
Call up Guinness World Records
One of the 10 fatal flaws in the original Corman-Drosten paper was that it was unclear whether it had ever been subjected to proper peer review – before, that is, the panel of experts took it upon themselves to do so. The paper had been submitted on January 22 and published the very next day. Peer review, when it takes place, is normally a long, drawn out process with plenty of back-and-forth, even when it is being rushed as much as possible. That it could be done in a single day beggars belief.
But that is what the authors are asking us to believe, as they are still claiming that their article was «peer-reviewed by two experts on whose recommendation the decision to publish was made.’’ Eurosurveillance may want to consider submitting this feat to Guinness World Records as the fastest peer review of all time – it may not be too late to get into the 2021 edition.
Also on rt.com
Landmark legal ruling finds that Covid tests are not fit for purpose. So what do the MSM do? They ignore it
In the United States, the official numbers currently show that 1.35 million people are confirmed as infected, while 80,351 people have so far died from the virus. If you take these numbers at face value, that would put the current Case Fatality Rate (CFR) for the coronavirus at 5.9%.
The infection numbers, though, are wildly over-inflated due to faulty testing kits that produce false positives. If we adjust the infection numbers down to a more realistic level, the CFR jumps significantly higher. And yes, there are likely some people dying from other things who have been incorrectly counted as COVID-19 deaths, but the Financial Times analysis of excess mortalities from all causes ends that argument by documenting a huge surge in recent deaths from any cause, regardless of what’s stated on death certificates.
If anything, the number of coronavirus deaths is being under-stated by perhaps 50% or so, while the number of coronavirus infections is being over-stated by a wide margin.
And we actually have a way to take a good guess at the degree by which those infection numbers are over-inflated.
We already know that many of these kits produce somewhere around 10 false positives per 100 people tested, or a 10% false positive rate (many kits are far worse). We can also intelligently estimate that right now somewhere around 2% of the US population has actually been infected. This is a rough estimate, but as you’ll see below, whether this is 1% or 4% doesn’t change the conclusions by much.
Now, if you test 100 people for the coronavirus, and 2 out of those 100 actually have the coronavirus, but the test kits you’re using have a false positive rate of 10 out of 100, then you will get, essentially 12 positives out of 100.
Notably, 10 of those positives are false, and 2 are real. This means the false positives are 500% higher than the real positives. And if you rely on those findings, you would incorrectly think that 500% more people have been infected than actually have.
This is precisely what the Stanford Study did. They ran tests that produced false positives, then they extrapolated that false finding to the entire population of California. From that, they incorrectly concluded that a huge percentage of California had already been infected, and therefore the Infection Fatality Rate (IFR) of the coronavirus was very, very small. As we show in this Natural News article, Stanford researchers likely produced 13 false positives for every 1 real positive.
That entire conclusion falls apart when you realize the testing kits they used were made in China. In fact, those particular kits were so unreliable that Stanford researchers tried to hide the origins of the kits in their paper, but internet sleuths found out the kits were actually made by Hangzhou Biotest Biotech, a company that ranked last place in testing kit accuracy. As ExtremeTech.com explained:
At the time Stanford did the study, there weren’t any FDA-approved COVID-19 antibody tests for clinical use. But for research purposes, the team purchased tests from Premier Biotech in Minnesota. Premier has started marketing a COVID-19 antibody test, but it doesn’t create it. The test listed on the company’s website, and that it appears Stanford used, is from Hangzhou Biotest Biotech, an established Chinese lab test vendor.
It also turned out that the Wall Street Journal writer who touted the stunning findings of the paper was one of the paper co-authors who failed to identify his obvious conflict of interest. So the entire study — and the subsequent WSJ editorial coverage of it — was a rigged scam, 100% science fraud parading around as breaking news to try to deceive America into thinking the coronavirus was no real danger at all. It was a propaganda con job, and sadly, most of the pro-Trump independent media fell for it and repeated the bad conclusions, misinforming their own audiences and causing many people to believe the virus was “no more dangerous than the flu.”
Peak Prosperity also explained this in a detailed video, which I covered in this important podcast:
If you really crunch the numbers on this, it turns out the coronavirus is 56 to 100 times more deadly than the regular flu. But to realize that, you have to weed through the deliberate disinformation being pushed by those who are trying to downplay the severity of the virus for political reasons. (A foolish ploy that will catastrophically backfire when the second wave of infections becomes impossible to deny.)
What about COVID-19?
In Australia, control measures have been very successful in reducing the number of people currently infected with COVID-19. We estimate the likelihood of a positive test to be very low right now (although of course this may change as restrictions ease).
The current reported number of active COVID-19 cases in Australia is about 600. And even if we’ve only diagnosed one in every ten people currently infected, this still represents less than 0.03% of the population.
Read more:
Can you get the COVID-19 coronavirus twice?
While we’re still establishing the specificity of tests for SARS-CoV-2 (the coronavirus that causes COVID-19), early evidence suggests an estimate of 99% or greater is reasonable.
However, following the same calculations as in the example above, at a prevalence of 0.03%, even a test with 99.9% specificity would mean only 30% of people who test positive actually have the condition. This means more than two-thirds of positive results would actually be false positives if we were testing asymptomatic people with no increased risk.
This is why testing criteria are often applied. If testing is offered only to those with symptoms consistent with COVID-19, the condition is almost certainly more common in those being tested than in the general (asymptomatic) population, and therefore the rate of true positives is going to be higher.
But if we start testing more broadly, the likelihood of false positives becomes a greater concern.
 Few new COVID-19 cases recorded from widespread testing is seeing restrictions beginning to ease in Australia.
Few new COVID-19 cases recorded from widespread testing is seeing restrictions beginning to ease in Australia.
Michael Dodge/AAP
A step-by-step example
Look at the following article to understand the same process in the context of a drug screening, which is exactly equivalent to the COVID-19 testing. This article goes through a numerical example and plots and charts to make the calculations clear and shows clearly how the characteristics of a particular test can impact the overall confidence in the test result.
Summary
The greatest global crisis since World War II and the largest global pandemic since the 1918–19 Spanish Flu is upon us today. Everybody is looking at the daily rise of the death toll and the rapid, exponential spread of this novel strain of the virus.
Data scientists, like so many people from all other walks of life, may also be feeling anxious. It may be somewhat reassuring to know that the familiar tools of data science and statistical modeling are very much relevant for analyzing the critical testing and disease-related data.
The goal of this article was to give an overview of some of the basic concepts in this regard. When you see a discussion about COVID-19 testing and its accuracy, you should be asking these questions and judge the result in light of data-driven rationality.
As this article points out, even the antibody or serological test suffers from the same limitation of false positives/negatives.
Medical professionals and epidemiologists work with this kind of analysis all the time. It is time that we also share this knowledge and understanding as much as we can and apply it rightly for discussion or decision-making.
Stay safe, everybody!
References
-
Petherick A. Developing antibody tests for SARS-CoV-2. Lancet 2020;395:1101-2.
-
- Watson J,
- Whiting PF,
- Brush JE
. Interpreting a covid-19 test result. BMJ2020;369:m1808.
OpenUrl
-
Kucirka LM, Lauer SA, Laeyendecker O, Boon D, Lessler J. Variation in false-negative rate of reverse transcriptase polymerase chain reaction-based SARS-CoV-2 tests by time since exposure. Ann Intern Med 2020;173:262-7.
-
Lind S. GPs to provide covid antibody testing for patients who have bloods taken. Pulse; 2020. http://www.pulsetoday.co.uk/news/gps-to-provide-covid-antibody-testing-for-patients-who-have-bloods-taken/20040894.article.
-
- Deeks JJ,
- Dinnes J,
- Takwoingi Y,
- et al.,
- Cochrane COVID-19 Diagnostic Test Accuracy Group
. Antibody tests for identification of current and past infection with SARS-CoV-2. Cochrane Database Syst Rev2020;6:CD013652.
-
Arevalo-Rodriguez I, Buitrago-Garcia D, Simancas-Racines D, et al. False-negative results of initial RT-PCR assays for covid-19: a systematic review. MedRxiv 2020.doi:10.1101/2020.04.16.20066787%J.</unknown>
-
Long Q-X, Tang X-J, Shi Q-L, et al. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat Med 2020;26:1200-4.
-
Whiting PF, Rutjes AWS, Westwood ME, Mallett S; QUADAS-2 Steering Group. A systematic review classifies sources of bias and variation in diagnostic test accuracy studies. J Clin Epidemiol 2013;66:1093-104.
-
- Ibarrondo FJ,
- Fulcher JA,
- Goodman-Meza D,
- et al
. Rapid decay of anti-SARS-CoV-2 antibodies in persons with mild covid-19. N Engl J Med2020;doi:10.1056/NEJMc2025179.
OpenUrlCrossRefPubMed
-
Public Health England. Sero-surveillance of covid-19. 2020. https://www.gov.uk/government/publications/national-covid-19-surveillance-reports/sero-surveillance-of-covid-19.
-
Gallais F, Velay A, Wendling M-J, et al. Intrafamilial exposure to SARS-CoV-2 induces cellular immune response without seroconversion. MedRxiv 2020.doi:10.1101/2020.06.21.20132449%J
-
Le Bert N, Tan AT, Kunasegaran K, et al. SARS-CoV-2-specific T cell immunity in cases of COVID-19 and SARS, and uninfected controls. Nature 2020;584:457-62.
-
Duan K, Liu B, Li C, et al. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc Natl Acad Sci U S A 2020;117:9490-6.
-
Wu F, Wang A, Liu M, et al. Neutralizing antibody responses to SARS-CoV-2 in a covid-19 recovered patient cohort and their implications. MedRxiv 2020.doi:10.1101/2020.03.30.20047365%J
-
Fierz W, Walz B. Antibody dependent enhancement due to original antigenic sin and the development of SARS. Front Immunol 2020;11:1120.
-
Hart JT. The inverse care law. Lancet 1971;1:405-12.
-
Andersson M, Low N, French N, et al. Rapid roll out of SARS-CoV-2 antibody testing-a concern. BMJ 2020;369:m2420.
-
Scottish Government. COVID-19 antibody testing 2020. 2020 https://www.gov.scot/news/covid-19-antibody-testing-1
-
Martin J. Covid-19 testing: a national strategy. Royal College of Pathologists 2020. https://www.rcpath.org/uploads/assets/2e8d8771-f85a-408a-b5c8e68969cd21d5/cbcb4f30-d8f8-40fe-ba8cdef3e6803ee4/RCPath-COVID-19-testing-a-national-strategy.pdf
-
Watson J, de Salis I, Banks J, Salisbury C. What do tests do for doctors? A qualitative study of blood testing in UK primary care. Fam Pract 2017;34:735-9.
-
Petrie KJ, Sherriff R. Normal diagnostic test results do not reassure patients. Evid Based Med 2014;19:14.
-
Rolfe A, Burton C. Reassurance after diagnostic testing with a low pretest probability of serious disease: systematic review and meta-analysis. JAMA Intern Med 2013;173:407-16.
-
- Weinstein MC,
- Freedberg KA,
- Hyle EP,
- Paltiel AD
. Waiting for certainty on covid-19 antibody tests—at what cost?. N Engl J Med2020;383:e37.doi:10.1056/NEJMp2017739.
OpenUrlCrossRef
-
Kofler N, Baylis F. Ten reasons why immunity passports are a bad idea. Nature 2020;581:379-81.
How false positives occur
Lagacé-Wiens says four false positives have been recorded in Manitoba to date.
«It is in a way bad luck, and it’s a fact of just the number of samples that we’re having to process in these automated instruments,» he said.
Across Canada, health workers use what’s called «reverse-transcriptase polymerase chain reaction» (PCR) testing to confirm cases of COVID-19. In several places, they’re also used to confirm when someone is recovered.
The tests evaluate a sample, taken with a swab of cells at the back of the nose and throat, for trace amounts of the coronavirus’s RNA.
This can be done one at a time, as with the GeneXpert technology used to double-verify presumptive cases in Nunavut, or in lab-built robotic testing machines, which can process hundreds of tests at a time.
In lab-built machines, robotic instruments extract pieces of hundreds of different samples and feed them into a PCR test. That’s where cross-contamination can happen.
A technician uses the Gene Xpert testing machine at Qikiqtani General Hospital in Iqaluit. The machine is now used to provide preliminary COVID-19 test results in the territory, before they are confirmed by southern labs. (CBC)
«Every now and again, even though it’s sort of a very precise robotic instrument … there can be very slight traces of carryover from sample to sample,» Lagacé-Wiens explained. «That’s where most of these false positives probably come from.»
Lagacé-Wiens said this most often happens when there is a very strong sample next to a negative one. The result is usually a very weak positive.
«If it’s a very, very weak positive, that’s usually your first hint that it merits rechecking. Another is that if a sample right beside it is strongly positive,» he said.
«Usually, when labs see that, they’ll go back to the original sample and they’ll re-test it,» he said.
Recommendations
The FDA recommends clinical laboratory staff and health care providers who use antigen tests for the rapid detection of SARS-CoV-2:
-
Be aware that the Conditions of Authorization in the antigen Emergency Use Authorizations specify that authorized laboratories are to follow the manufacturer’s instructions for use, typically found in the package insert, when performing the test and reading test results. If you no longer have the package insert for the test you are using, you can contact the manufacturer. The authorized instructions for use for each test can also be found on the FDA’s COVID-19 IVD EUA webpage.
- For example, the package insert for tests include instructions for handling of the test cartridge/card, such as ensuring it is not stored open prior to use. If the test components are not stored properly, this can affect the performance of the test.
- The package insert for tests also includes instructions about reading the test results, including the appropriate time to read the results. Reading the test before or after the specified time could result in false positive or false negative results.
- Be aware that processing multiple specimens in batch mode may make it more challenging to ensure the correct incubation time for each specimen. Refer to the package insert and ensure proper timing for each specimen when processing the specimen in the test device and reading the results.
- Be careful to minimize the risks of cross-contamination when testing patient specimens, which can cause false positive results. Insufficient cleaning of the workspace, insufficient disinfection of the instrument, or inappropriate use of protective equipment (for example, failing to change gloves between patients) can increase the risk of cross-contamination between specimens with subsequent false positive results. Consider the CDC guidance for changing gloves and cleaning work area between specimen handling and processing.
- Consider the CDC’s recommendations when using antigen testing in nursing homes and other settings. For positive results, especially in low incidence counties, consider performing confirmatory RT-PCR test within 48 hours.
- Remember that positive predictive value (PPV) varies with disease prevalence when interpreting results from diagnostic tests. PPV is the percent of positive test results that are true positives. As disease prevalence decreases, the percent of test results that are false positives increase.
- For example, a test with 98% specificity would have a PPV of just over 80% in a population with 10% prevalence, meaning 20 out of 100 positive results would be false positives.
- The same test would only have a PPV of approximately 30% in a population with 1% prevalence, meaning 70 out of 100 positive results would be false positives. This means that, in a population with 1% prevalence, only 30% of individuals with positive test results actually have the disease.
- At 0.1% prevalence, the PPV would only be 4%, meaning that 96 out of 100 positive results would be false positives.
- Health care providers should take the local prevalence into consideration when interpreting diagnostic test results.
- Consider positive results in combination with clinical observations, patient history, and epidemiological information.
- Be aware that the Conditions of Authorization in the antigen EUAs specify that Authorized Laboratories are to collect information on the performance of antigen tests and report any suspected occurrence of false positive or false negative results and significant deviations from the established performance characteristics of which they become aware to both the FDA and the test manufacturer.
Types of tests
Two types of COVID-19 tests are available: Diagnostic tests and antibody tests.
A diagnostic test can show if you have an active coronavirus infection. Currently there are two types of diagnostic tests: viral PCR tests that detect the virus’s genetic material, and antigen tests that detect specific proteins on the surface of the virus. Testing sites listed on this page use viral PCR tests.
Antigen tests usually provide results faster than viral PCR tests at lower cost, but have a higher chance of missing an active infection. Antigen tests are used on people suspected of having COVID-19 within 5-12 days of symptoms appearing.
Antibody tests detect past infections. They can determine if you are a good candidate to donate blood plasma. It can take 1-3 weeks after infection for your body to make antibodies.
You can find locations for both viral PCR and antibody tests on the COVID-19 Testing Sites in California map.